Menu
Quality control is required throughout the entire development process, from drug substance and raw material control, through intermediate drug product testing, to finished drug product release testing, bioavailability and stability studies. By having a comprehensive understanding of the physical and chemical properties of your drug substances and drug products, you can develop the most appropriate drug product formulations. Nikyang represents the pharmaceutical testing leader, SOTAX in providing the testing equipment in the development and production processes for our customers in the pharmaceutical industry. Our extensive market and process know-how enable us to meet our customers’ requirements and comply with the USP, EP and CP requirements relevant to them. We are also open to cooperation with new industries.
Dissolution

AT - A fully scalable and modular dissolution sampling apparatus
Incorporating the concept the modular, scalable and future-proof technologies, AT dissolution apparatus provides a complete and flexible sampling solution in R&D and QC laboratories.

AT 70smart - Fully automatic dissolution tester
For dissolution testing, the AT 70smart is a fully automatic device that permits fast and efficient running of sequence testing of 10 (USP 1) or 15 (USP 2) batches. It is compliant with pharmacopeia methods including USP Apparatus 1 (basket), Apparatus 2 (paddle), Apparatus 5 (paddle over disk), Apparatus 6 (rotating cylinder), Intrinsic Dissolution, Stationary basket and Mini Paddles/Baskets/Vessels. It also fulfils all related EP, JP and CP requirements.

CE 7smart - Flow through cell Dissolution Tester
The CE 7smart is the 4th generation of Sotax's design for dissolution of poorly soluble and release control dosage forms via USP Apparatus 4 (Flow-Through Cell Dissolution), and is compliant to USP, EP and JP for Small Volume Dissolution and Poorly Soluble Compound Testing under Sink conditions. Based on 35 years of experience, the CE 7smart meets USP requirements for the flow rate and temperature to be qualified, and is designed to overcome potential challenges linked to method development for a variety of dosage forms.

DVC-24 Dissolution Vessel Cleaner
Robust, mobile, and easy to use, the DVC-24 is an automated dissolution vessel cleaner which is adaptable to all types of dissolution testers. It empties and effectively cleans multiple dissolution baths with a patented steam-cleaning process.
Physical Testing

DT50 - Automatic Tablet Disintegration Tester
DT50 automates the tablet disintegration process in full compliance with current Pharmacopeia regulations. The compact and fashionable wireless design occupies a small footprint but carries all optimized functions in a user-friendly platform.

DT2 - Manual Disintegration Tester
The DT2 is a very easy-to-use and flexible disintegration tester with reliable temperature control. It records and reports disintegration times individually per sample or as a completed set. Two stations can be controlled, started, and stopped individually and allow performing 2 tests independently.

FT2 - Friability Tester
This microprocessor-controlled automated tablet friability testing device for single or dual drums is compliant with USP and EP requirements. Featuring convenient front loading of tablets and automatic discharge after test completion, the FT2 is easy to use with an LCD display and scroll-down menus, and flexible operation including timer, counter or speed modes.

PF1 - Powder and Granulate Flowability Tester
The PF1 powder flow tester has been specifically designed for standardized testing in full compliance with Pharmacopeia requirements as laid down in USP <1174> and Ph. Eur. 2.9.36. Its optimized design allows for different, yet easily repeatable test conditions and methods for powder flow characterization. Change-over between configurations is simple with quick-change components that do not require any additional tools.

ST50 - Semi-automatic Tablet Hardness Tester
Tablet testing has become much easier with the ST50 now. The integrated SmartAlign™ system sets new standards in fast and efficient testing of up to 5 physical parameters (weight, thickness, width, diameter / length, hardness). It also allows correct positioning for samples at different shapes, offering maximum flexibility for diverse laboratory and production environments.

MT50 - Manual Tablet Hardness Tester
It becomes much easier to perform tablet hardness test with MT50. By integrating advanced features, you can now enjoy simplicity, efficiency even from a manual operation.

AT50 - Fully Automatic Tablet Hardness Tester
SOTAX AT50 is a fully automated tablet testing device, and well-known for its high-speed, reliability, precision and ease of operation. It features automated measurement of weight, thickness, width, length / diameter and hardness of loading up to 10 different products. Equipped with both AutoAlign™ and SmartAlign™, the AT50 offers reliable orientation of all tablet shapes for repeatable results, including difficult-to-orient oblongs, oval tablets, and even unconventional convex or flat shapes.

TD1 - Tap Density Tester
SOTAX TD1 comes with new housing in contemporary silver design. It measures the tapped density and tapped volumes of powders, granules, pellets, flakes and other bulk substances according to EP, ASTM and USP regulations. This technique is useful in powder compression and flowability studies as well as to determine the amount of settlement during transit and packaging.

TM200 - Cap Torque Tester
Proven torque tester for repeatable measuring of opening & closing torque of bottle caps including child-resistant closures. Custom-made cap adapters avoid undesired effects of clamping or applying additional pressure on bottle caps during measuring. A motorized drive ensures repeatability and highly accurate turning movements.
Sample Preparation

TPW - Automated Tablet Processing Workstation
The TPW Tablet Processing Workstation from Sotax performs fully automated sample preparation and analysis for the most common pharmaceutical tests in Development and Quality Assurance functions, such as Stability and Uniformity testing. The TPW is specifically designed to prepare and analyse pharmaceutical solid dosage forms and intermediate granulations.
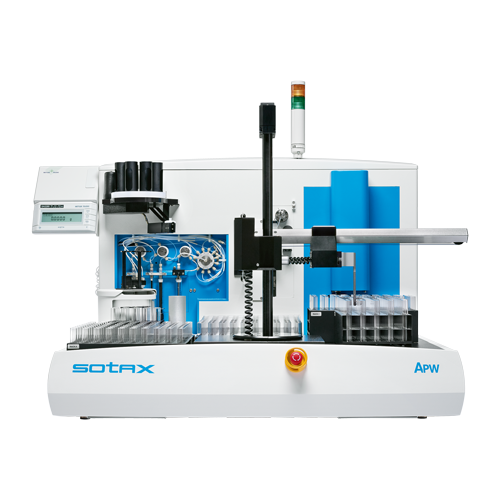
APW - API Workstation
The APW Active Ingredient Processing Workstation from Sotax performs fully automated sample preparation and analysis for the most common Active Pharmaceutical Ingredient (API) tests in pharmaceutical Development and Quality Assurance functions, such as Stability and Uniformity testing. Fully automated sample preparation provides fast, consistent and accurate analytical results, greatly enhancing work efficiency.

MPS - Media Preparation Station for dissolution media
When USP 1,2,4,5 & 6 dissolution tests are carried out, the preparation of the media and its degassing, tempering and accurate metering into the test vessels involves substantial effort. The Sotax MPS (Media Preparation Station) automates these tasks, permitting the efficient preheating and degassing of the dissolution media using Sotax Thin Film Technology™, as well as fast and accurate filling. For even greater efficiency, the use of concentrates allows the system to further reduce media preparation time.
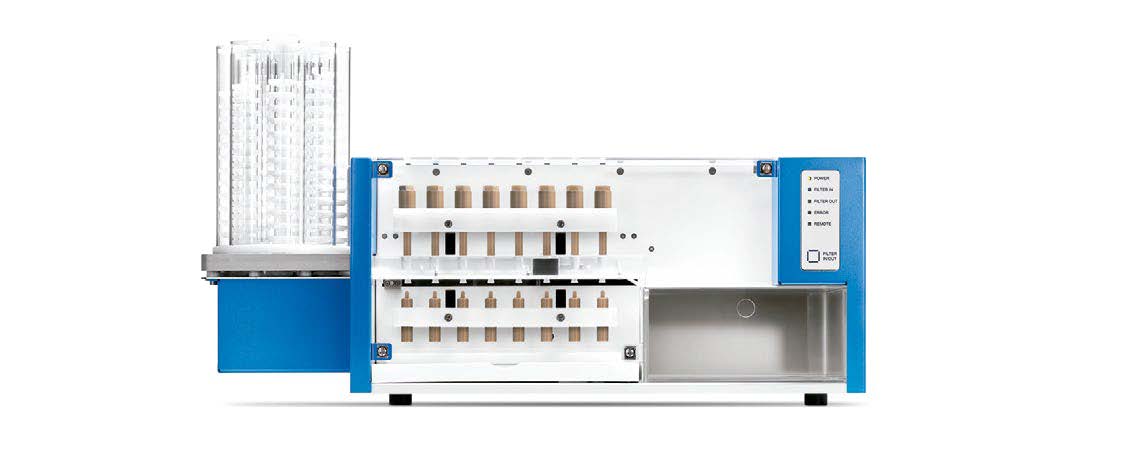
FS - Automated filter change workstation
Suitable for all SOTAX dissolution systems, this automated filter workstation automates filter changes after each sampling point for extended testing and media change methods. The standalone FS provides reliable filter changes and solves the filter clogging and saturation problems commonly associated with 25 mm diameter filters.